Completing a Master’s in Pharmaceutical Care or Drug Development opens up a world of possibilities. You might have considered working in a pharmacy, but that’s just the tip of the iceberg. Depending on your interests, various career paths await, each leading to different job opportunities (see Figure 1).
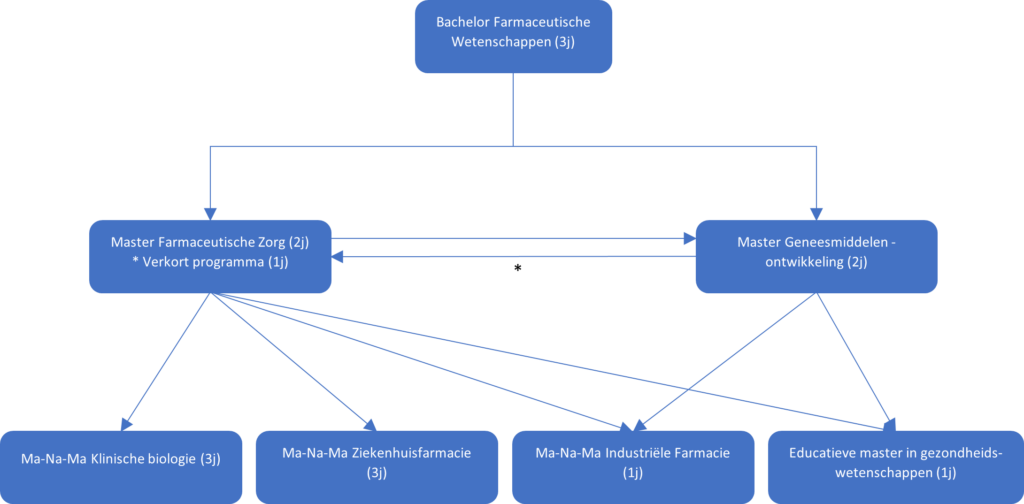
If you’re exploring a career beyond the (hospital) pharmacy, it can be challenging to know where to start. During the Master’s in Drug Development, you get a broad overview of a drug’s journey and the organizations involved, such as (bio)pharmaceutical/technological companies, analytical laboratories, clinical investigational sites, and more. But, this landscape is vast, and the guidance can sometimes be, “You can go anywhere; choose what you want to do,” which may not provide much clarity.
As a recent graduate in pharmaceutical sciences, you may not know what roles to pursue or where to apply. Positions like QA Officer, MSL, CRA, etc., might sound unfamiliar, let alone understanding their responsibilities.
So, here are some examples of career options after graduating with one of the master’s degrees mentioned in Figure 1.
Qualified Person (QP)
As a QP, you hold the ultimate responsibility for batch certification and release, following the guidelines outlined in Annex 13 and Annex 16 of the Eudralex Vol. 4. [1]
You prepare a release dossier containing all pertinent documents related to the batch to be released. These documents undergo two types of reviews: 1) Good Manufacturing Practices (GMP) compliance and 2) Regulatory Compliance.
Depending on your review, a medicine is either released or rejected. In the latter case, it means the medicine won’t reach the pharmacy/clinical investigational site, and ultimately, the patient.
Documents that may be part of the release dossier include the Certificate of Analysis (CoA), label pictures, release statements, deviation reports, change control reports, SAP traceability data, IMPD, SmPC, and more.
Being a QP requires critical thinking and an understanding of the entire supply chain for the batch, as well as staying updated on pharmaceutical regulations.
- For more information, you can always reach out to Farma Consulting & Partners.
- A Master’s in Industrial Pharmacy is a minimum requirement.
Quality Assurance Officer (QA Officer)
As a QA Officer, you are responsible for creating, maintaining, and optimizing a Quality Management System (QMS). This ensures quality and compliance with Good Manufacturing Practices (GMP), other regulations, and internal procedures. Your tasks include writing/updating standard operating procedures (SOPs), conducting internal audits, hosting external/health authority audits, providing training (including annual GMP training), supplier/vendor qualification, handling non-conformities (deviations, OOS, complaints), and implementing Change Management & CAPAs using a risk-based approach.
Additionally, QA Officers can support QPs in preparing a release dossier.
- For more information, you can always reach out to Farma Consulting & Partners.
Quality Control Analyst (QC Analyst)
As a QC Analyst, you primarily work in the lab. You gain experience in performing various analytical techniques (such as (U)HPLC, Flow Cytometry, GC, qPCR, CE, DSC, IR spectroscopy, Raman, XRD, KF titration, etc.), making calculations, interpreting and reporting results, and reviewing others’ analyses. Training newcomers and contributing to the optimization of certain analytical methods may also be part of your duties.
You’re likely to work in a collaborative lab environment where everyone is ready to help each other.
Regulatory Affairs Officer (RA Officer)
As an RA officer, you oversee the Life Cycle Management of a medicine. It starts with obtaining a Marketing Authorization (MA), which is a dynamic process. Continuous changes can occur in dossiers due to regulatory requirements or new scientific information.
Using “variations,” you’ll make these changes in the dossiers, including product information (patient leaflet, scientific information for healthcare professionals, and packaging texts), following evaluation and approval by health authorities.
RA Officers liaise with health authorities and internal colleagues, such as market access and QA.
RA ensures that the RIGHT information reaches the patient.
“There will come a time when you believe everything is finished. That will be the beginning”
- For more information, you can always reach out to Anke Nijst.
Pharmacovigilance Officer (PV Officer)
This role primarily involves collecting and documenting PV information (e.g., adverse events experienced by individuals after taking a medicine) in line with European drug monitoring regulations.
Pharmaceutical companies receive information about adverse events in various ways: spontaneous reports from patients, visits by MSLs or sales representatives to doctors, patient support program follow-ups, or screening scientific articles.
Once PV information reaches the pharmaceutical company, PV officers create an Individual Case Safety Report (ICSR) within a specified timeframe. This report is then sent to health authorities.
PV officers are also responsible for training colleagues in other departments on their reporting responsibilities.
- For more information, you can always reach out to Julie Van Dyck.
Clinical Research Associate (CRA)
As a CRA, you’re responsible for initiating, supervising, and monitoring clinical studies in hospitals. You train and guide doctors and nurses through the clinical study, ensuring they follow protocol-specific procedures.
It’s a job that involves being on the move, interacting socially, and using your medical knowledge to interpret patient records, attend consultations, and engage in discussions with doctors.
- For more information, you can always reach out to Birte De Jongh.
Brand Manager
As a Brand Manager, you ensure the creation of materials (with input from doctors) for sales representatives. You receive an annual budget for materials, organizing webinars, specific commercial campaigns, and value-based healthcare projects. It’s essential to align these activities with the strategy and vision of your product Quarterly performance reviews and an annual brand plan also come into play. Quarterly performance reviews and an annual brand plan also come into play.
- For more information, you can always reach out to Isabelle Schollaert.
- A General Management diploma from Vlerick Business School can be an added advantage.
Health Economics and Market Access Specialist (HEMA Specialist) – Within the Medical Devices Industry
As a HEMA Specialist, you work on increasing patient access to innovative medical devices in Belgium by obtaining reimbursements for these devices. This involves demonstrating the added clinical and economic value to society, hospitals, healthcare professionals, patients, and their families, as well as engaging the right internal and external stakeholders.
Besides securing reimbursements, you analyze transformations in the healthcare system (e.g., the shift from “fee for service” to “DRG” [2] in hospital financing) and their implications for medical device access. This often involves collaboration with the entire medical devices industry through trade associations (beMedTech).
- For more information, you can always reach out to Laura Vertommen.
And Many More Opportunities
Of course, this is just a limited list. We haven’t even touched on roles like Medical Science Liaison (MSL), Sales Representative, Scientist, Group/Team Leader, Medical Affairs, etc. Let alone positions outside the (bio)pharmaceutical industry, such as pursuing a Ph.D. or policy roles within RIZIV, FAGG, and more.
Numerous job opportunities await, so be sure to engage in conversations with others! At Farma Consulting & Partners, we connect with various companies and roles, so feel free to reach out if you’d like more information!
[1] Eudralex Vol. 4 contains guidelines for interpreting “Good Manufacturing Practice guidelines for medicinal products” for human and veterinary use.
[2] Hospitals are no longer paid per activity but per admitted patient with a specific diagnosis.
Written by Lien Secretin
Credits to Anke Nijst, Birte De Jongh, Isabelle Schollaert, Julie Van Dyck en Laura Vertommen.
