In this blog, a high-level introduction is given with respect to technology transfer in pharmaceutical manufacturing.
Technology transfer is the collaborative process for the directed dissemination of scientific findings, knowledge and intellectual property from the inventors to a receiving party. Specifically in pharmaceutical industry, it plays an important role within product life cycle management, from drug discovery to industrial scale pharmaceutical manufacturing.
Technology transfer can be put in place between different knowledge institutes/companies, or within a company, and this between the same or different phases in the pharmaceutical drug substance/drug product development (for example transfer between R&D – R&D, R&D – manufacturing, or manufacturing – manufacturing sites). The transfer can include also scale up / optimizations / any other changes (e.g. equipment design / materials (suppliers) / control strategy / …) of the process being transferred, and can involve differences in regulation (since the transfer can involve different countries).
In order to comply to all regulatory expectations, such as those described under the pharmaceutical quality management system, the technology transfer needs to be based on documented knowledge/experience and its successful completion needs to be evidenced at the receiving side in their ability to effectively perform and control all critical aspects of the transferred technology showing they are able to produce quality product consistently at the defined manufacturing scale. For this a systematic approach to technology transfer needs to be followed and approved by both parties, as described in a Technology Transfer (master) Plan. The general steps you will likely encounter during a technology transfer project are shown below and are discussed further in this blog.
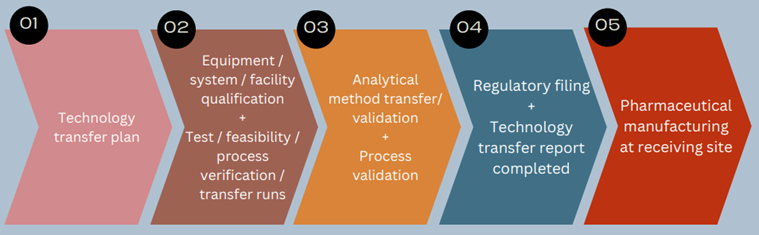
Step 01
The Technology Transfer Plan needs to give a detailed description of all documentation regarding the technology (product, process or procedure) in scope and the activities that need to be delivered/performed, along with a schedule, roles and responsibilities and acceptance criteria for the transfer from the perspective of both parties.
Below a handy checklist is provided of the documentation/knowledge a transferring site typically needs to have regarding the process and will need to provide and explain to the receiving site. The level that the information is defined/proven should be commensurate with the stage within product life cycle (it should be higher the later in the life cycle stage).

Apart for an approved comprehensive Technology Transfer Plan the success of the technology transfer will be greatly facilitated by setting up and continuously maintaining clear communication between the transferring (or sending) site and receiving site, preferably having a cross-functional project team at both sites dedicated to the technology transfer.
Changes, delays, failures, risks, findings,… with possible impact on any aspect in scope of the technology transfer should be openly communicated between both parties, in order to assess and if required implement correctly and in time the required mitigations/actions. Standard procedures should be in place to cover this.
An important part of the technology transfer is performing a technology transfer gap-fit assessment and based on it define control/mitigation actions to overcome any differences at the receiving site versus the transferring site. The assessment needs to include differences in intended batch sizes, premises, equipment, instruments, procedures, quality management, technical capabilities, qualification and validation expertise, personnel, health & safety and environmental regulations, materials, suppliers,…
Step 02
Before performing the process validation runs at the receiving site, it is required to complete the commissioning and qualification of the facility and equipment. And it is also likely needed to perform test / feasibility / process verification / transfer runs based on study plans in order to gather the required information on the impact of any change introduced along with the transfer, to allow for the assessment of improvements/optimizations, and to assess the overall process readiness at the receiving site.
Step 03
Since analytical methods are needed from the onset of the studies to provide data and demonstrate the results of the (comparability) studies, it is required to make sure their transfer and/or validation at the receiving site is included in the early phase of the technology transfer. This needs to span the testing needed on the input materials, in-process samples, cleaning samples and the end product(s) in accordance to their release specifications.
Successful transfer of the pharmaceutical manufacturing process will be evidenced by the successful completion of the assessed required number of process validation runs (in general 3 consecutive successful runs is accepted as the standard). Here the receiving site’s ability will be tested to perform and control the process consistently resulting in end product with the predefined quality attributes. Apart from process validation, cleaning validation, stability, hold-time, bioburden and extractable & leachable studies might be required to be done as well at the receiving site.
For the qualification and validation activities to be successful in addition to the criteria specific to the transfer, it is required to have a robust quality management system in place (which entails training management, deviation management, change management, risk management,…).
Step 04
The progress and success of the technology transfer needs to be monitored and reviewed throughout the project and at the end of the transfer a Technology Transfer Report should be made to conclude if the technology transfer has been successfully completed, by verify all activities and acceptance criteria as listed in the Technology Transfer Plan have been met, and/or any changes or deviations have been investigated and mitigated accordingly.
In conjunction with the regulatory affairs teams the technology transfer team will need to prepare the regulatory submission package, this is best planned early on so it is ready for submission soon after the completion of the process validation.
Step 05
After approval of the regulatory filling of the transferred process by the receiving site the commercial production can be initiated at the receiving site. From here the process is included in the site’s ongoing process validation and continuous improvement activities
Suggested further reading:
- Annex 4 WHO guidelines on technology transfer in pharmaceutical manufacturing
- PDA Technical Report No.65 Revised 2022 (TR 65) Technology Transfer
Relying on a partner that has experience with supporting technology transfers can prove to be a big factor in both the speed and successfulness of your company’s technology transfers. Farma Consulting & Partners can provide your company with consultants that can facilitate and/or lead your technology transfer projects, contact us today for more information!
